Do vibrationally excited OH molecules affect middle and upper atmospheric chemistry?
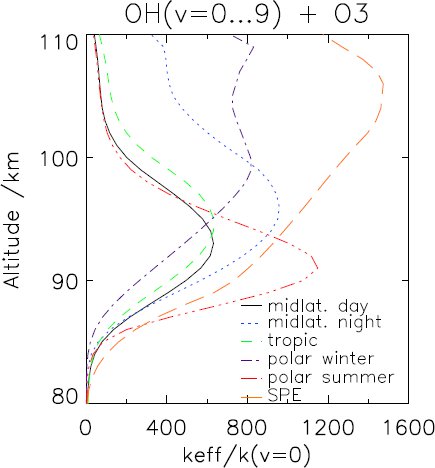
Above 80 km the vibrational states of OH are populated much more than under thermodynamic equilibrium conditions. Due to this, the reaction OH+O3 → O2+HO2 can be up to a factor of nearly 1500 faster than under thermodynamic equilibrium conditions. The implication of this on odd oxygen chemistry has been assessed.
At altitudes where non-local thermodynamic equilibrium is important, OH+O3 → O2+HO2 is only a minor sink of ozone. Thus, the effect of vibrational excitation of OH on atmospheric chemistry remains small. Although the dependence of the reaction rate of OH+O → O2+H on the vibrational states of OH is smaller, its effect on atmospheric chemistry is larger. The OH sink is overestimated by up to 10% for quiescent atmospheres and 12% after a solar proton event.
For details, see: von Clarmann et al., ACP 10, 9953–9964, (2010), doi:10.5194/acp-10-9953-2010.
